My brain is currently in soap and lye mode. Between having the honour to judge a lye experiment at Gulf Wars A&S War point (If you wrote that paper, I’d love a copy please! If you know the good gentle who wrote it, please nudge them to share it!), planning to teach saponification at the University of Atlantia in June and also planning to teach soap making at Fruits of Our Labours in May.. well there’s a lot of soap and lye thoughts running around in my brain.
Historically lye is produced by running water through hardwood ashes, to get a very alkaline potassium solution. Lye is not a specific term, but a general term that can refer to sodium hydroxide or potassium hydroxide. What we’re aiming for is that the potassium carbonate (potash) that is in the ashes to react with the water we’re providing to give us potassium hydroxide. Modern lye is produced from pure sodium hydroxide crystals being hydrated in water, but the process to refine sodium hydroxide into pure crystal form wasn’t established until about 1790, which is solidly outside SCA period. If you are fortunate enough to live where you have marine (not lakes.. has to be salt water here) vegetation to burn for ash, you are likely to get enough sodium for your lye to be mostly sodium hydroxide, which made (and makes) nice firm bars of soap. Soap made using potassium hydroxide tends to never really get firmer than play-doh sort of solid. It washes fine, but it’s not a good solid bar of soap.

Generally I get my ashes from a good friend with a wood fired pizza oven, we don’t have a fire place or a wood stove of our own and honestly the bucket I got from him about 3 or 4 yrs back is still mostly full, so I don’t need a refill all that often. That being said, that is not always completely burnt wood, there’s a lot of chunks of charcoal left, and half burnt wood. It’s not a fine white ash of utterly burnt. It works alright after sifting the worst of the chunks out, but it’s not ideal. Leaving chunks of charcoal in your ashes lets charcoal do what it does best, filter things. Including the lye you are trying to make, so you want to take those chunks out.
Yesterday was the first perfect day of spring around here. Warm, sunny and perfect, so we decided to grill burgers for supper. We’re those weirdo folks who have a charcoal grill and after dinner, I was eyeing all that lovely white ash. I have no idea what commercial charcoal is made from, but I decided to operate on the theory that it was probably hardwood and give it a test this morning. For the record, coals about 18 hrs later are still hot enough to melt your plastic colander. I switched to the metal sieve after that.

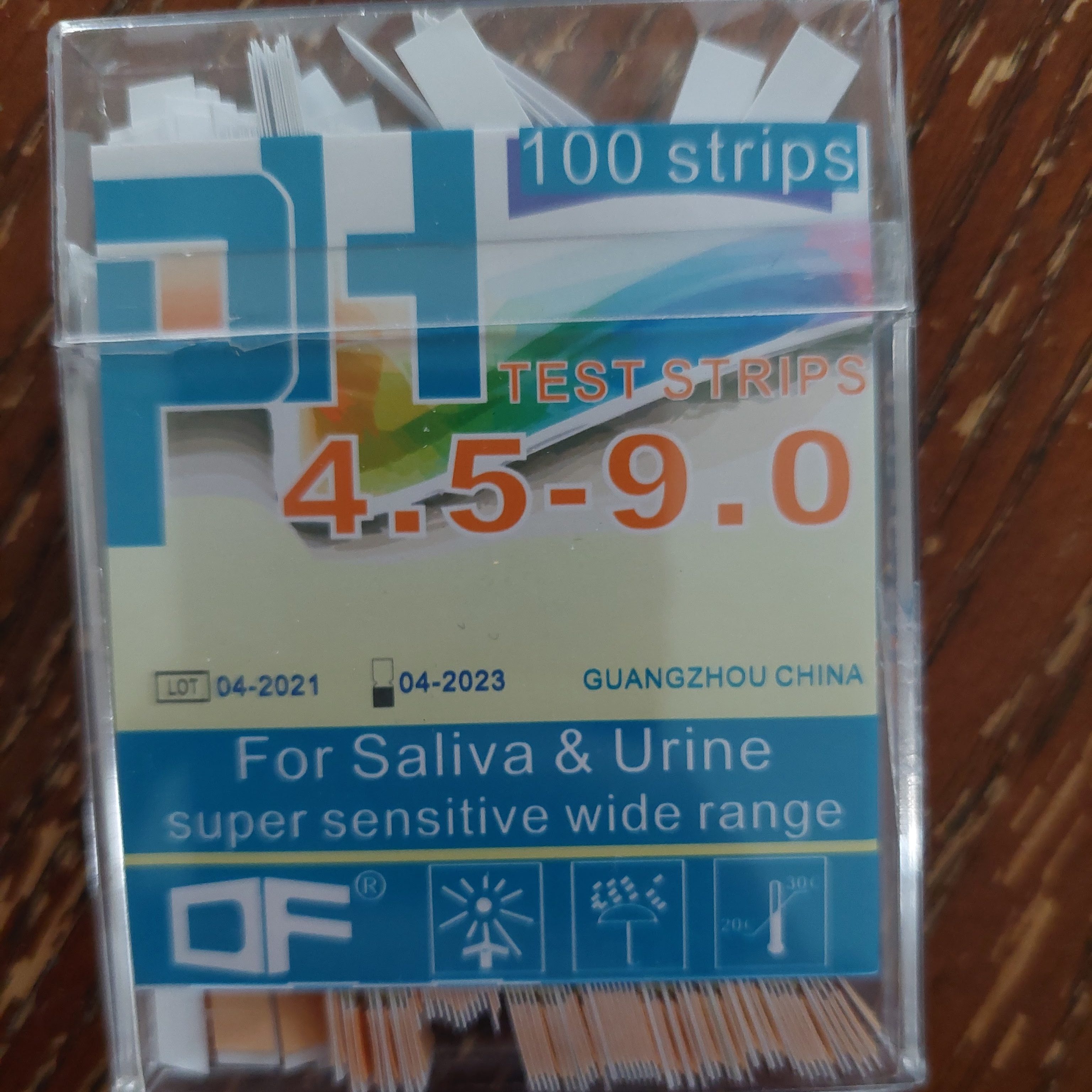
While just letting ashes soak is not my preferred lye making method, its good enough for a proof of concept. Traditionally you want the water to run through the ashes and be filtered through straw at the bottom of your barrel, rather than a messy sludge of ashes and lye. (Or in my case, the plastic ice cream bucket, and my straw filter layer is a small piece of linen over the hole). I also don’t have ideal pH strips for lye experiments, as mine stop at 9 and lye is in the 13 – 14 range. I wanted these to be more specific over the neutral range, rather than have a wider range. I’ll have to pick up the wider range ones at some point. Still, proof of concept here, and success! The water sat for an hour or so at most, and its reading at least 9 on the pH scale. Found a new source of ash for experiments!